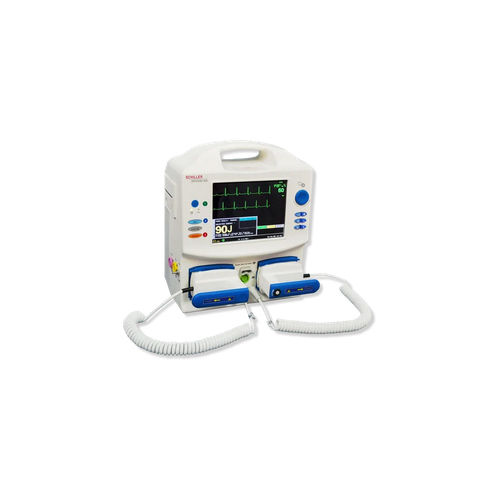
Schiller Defigard 400 Biphasic Defibrillator
164000.0 INR/Piece
Product Details:
- Product Type Schiller Defigard 400 Biphasic Defibrillator
- Display Digital
- Feature Good Quality
- Condition New
- Size Standard
- Used For Hospital
- Origin India
- Click to view more
X
Schiller Defigard 400 Biphasic Defibrillator Price And Quantity
- 164000.0 INR/Piece
- 1 Piece
Schiller Defigard 400 Biphasic Defibrillator Product Specifications
- Digital
- Schiller Defigard 400 Biphasic Defibrillator
- Standard
- New
- Good Quality
- Hospital
- 1 Year
- India
Schiller Defigard 400 Biphasic Defibrillator Trade Information
- 10 Piece Per Month
- 7 Days
- Punjab, Delhi, Haryana, Rajasthan, Uttar Pradesh
Product Description
| Model Name/Number | Defigard 400 |
| Category Type | defibrillator |
| Type | Biphasic Defibrillators |
| Brand | Schiller |
Additional Features:
- Trend storage for the last 11 defibrillation event groups.
- Storage capacity for 3 defibrillation strips.
- 24 hours of graphical and tabular trend data for all parameters.
- Internal defibrillation capability through spoon paddle.
- Optional features include AED mode, external pacemaker with fix, demand, and overdrive modes, 12-lead ECG, SpO2, NIBP, EtCO2.
- Integrated thermal printer with auto and manual modes of printing.
- USB interface for convenient data storage.
Reliable Emergency Cardiac Care
Equipped with advanced biphasic technology, the Schiller Defigard 400 delivers consistent and safe defibrillation. Hospitals benefit from its intuitive digital display and ergonomic design, helping medical personnel respond confidently during emergencies. Its high manufacturing standards and Indian origin make it trusted among exporters and suppliers.
User-Friendly Digital Display
The defibrillators digital display shows vital information with clarity, facilitating accurate decision-making for healthcare professionals during resuscitation. The standard size ensures easy integration into hospital environments, and the overall durable build supports long-term usage.
FAQs of Schiller Defigard 400 Biphasic Defibrillator:
Q: How does the Schiller Defigard 400 Biphasic Defibrillator improve patient outcomes in hospitals?
A: The biphasic defibrillation technology delivers effective shocks with reduced energy, minimizing heart tissue damage and increasing the chances of successful resuscitation during cardiac emergencies.Q: What is included in the warranty for the Schiller Defigard 400, and how long does it last?
A: The product comes with a one-year warranty that covers manufacturing defects and malfunctions under normal use, providing peace of mind to hospitals and medical suppliers.Q: When should the Schiller Defigard 400 be used in a hospital setting?
A: It should be used during cardiac arrest situations where immediate defibrillation is required, including emergencies in emergency rooms, intensive care units, and ambulance setups.Q: Where is the Schiller Defigard 400 manufactured, and who can source it in India?
A: The device is manufactured in India and is available through exporters, importers, manufacturers, suppliers, and traders catering to healthcare institutions across the country.Q: What is the process for installing and using the Schiller Defigard 400 in a hospital environment?
A: The defibrillators standard size allows straightforward installation on crash carts or designated medical stations. It is powered on and operated via its digital interface, which guides users through electrode placement and shock delivery.Q: What are the primary benefits of choosing the Schiller Defigard 400 Biphasic Defibrillator?
A: Hospitals gain a reliable, high-quality device with modern biphasic technology, clear digital display, and robust warranty support, ensuring effectiveness in critical care situations.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email